Stanley MeyerHHO
#Hydrogen #Electric #Generator

Stanley Meyer's water fuel cell - Wikipedia, the free encyclopedia
Nafion Inline Filters and Tubes
Secure Supplies manufactures components and devices primarily designed to dry and humidify gas streams.
Our core technology employed is Nafion®, a Dupont co-polymer that is highly selective in the removal of water. We manufacture Nafion into tubing of various diameters to optimize the transfer of water through the Nafion.
The water moves through the membrane wall and evaporates into the surrounding air or gas in a process called perevaporation. This process is driven by the humidity gradient between the inside and the outside of the tubing. In addition to drying and humidifying, our products can be used as ion exchange membranes and specialty separation membranes that take advantage of the unique properties of Nafion.
Nafion – The Perfect Drying and Humidifying Material
Nafion is a copolymer of tetrafluoroethylene (Teflon®) and perfluoro-3,6-dioxa-4-methyl-7-octene-sulfonic acid. Like Teflon, Nafion is highly resistant to chemical attack, but the presence of its exposed sulfonic acid groups confers unusual properties. Sulfonic acid has a very high water-of-hydration, absorbing 13 molecules of water for every sulfonic acid group in the polymer; consequently, Nafion absorbs 22% by weight of water.
Unlike micro-porous membrane permeation, which transfers water through a relatively slow diffusion process, Nafion removes water by absorption as water-of-hydration. This absorption occurs as a First Order Kinetic reaction, so equilibrium is reached very quickly (typically with in milliseconds). Because this is a specific chemical reaction with water, gases being dried or processed are usually entirely unaffected.
Nafion tubing is very stable at high temperatures. As noted previously, its chemical resistance is similar to Teflon, and very few things can attack it. Even samples containing high concentrations of very corrosive gases like hydrogen fluoride or hydrogen chloride can be dried or processed. In addition to the Nafion tubing, most dryers and humidifiers include a shell to contain the purge gas as well as fittings for gas connections to the gas stream and purge lines. Shells and fittings are available in stainless steel, fluorocarbon polymer, or polypropylene.
For higher gas stream flow rates, bundles of Nafion tubing are formed using chemically resistant thermosetting epoxy resin. The temperature resistance properties of the dryers are dependent on these other materials as well. The gas stream does not come into contact with the shell but the fittings for gas stream connection do contact the gases.
For our single tube gas dryers, the maximum temperature for polypropylene shells and fittings is 100°C, and 150°C for fluorocarbon and stainless steel. When Nafion tubing bundles are used, the epoxy limits the maximum temperature to 120°C. The maximum internal pressure for all dryers is usually limited by the Nafion tubing to 80 psig at 20°C. Recent advances in some specialty dryers have increased this pressure tolerance.
Dryers and humidifiers with single strands of Nafion tubing tolerate any gas stream that its fittings tolerate. Dryers and humidifiers with Nafion tubing bundles are somewhat more sensitive due to the epoxy resin used to channel the gas stream into each tube. technical staff will verify the acceptability of highly corrosive sample


Proven Technology
Du Pont Developed the first Nafion dryers more than thirty-five years ago. Today dryers are used throughout the world for gas sample conditioning prior to analysis by medical, scientific, and industrial instrumentation.
This drying technology is branching out from the sampling environment into the process world, with applications ranging from medical drying to industrial process drying of various gases. Humidifiers are also evolving from the analytical and laboratory arena to function as specialty humidifiers in applications ranging from medical gas humidification to humidifying hydrogen for fuel cells. In every application, products are guaranteed to perform reliably every time.
Nafion Application Information
-
Effects of Temperature on Nafion Gas Dryers
-
Compounds Removed by Nafion Dryers
-
Sulfuric Acid in Gas Samples
-
Drying Technology: Microporous vs Nafion
-
Nafion: Physical and Chemical Properties
-
Tap Water and The Ion Exchange Properties of Nafion
-
The Acidity of Nafion
-
Formaldehyde Permeation Through Nafion
-
Gas Permeability of Nafion
-
Read on below thanks for visiting warm regards Dan !!
Frequently Asked Questions About Nafion
What is Nafion?
Nafion® is a copolymer of perfluoro-3,6-dioxa-4-methyl-7octene-sulfonic acid and tetrafluoroethylene (Teflon®). In simpler terms Nafion is a Teflon backbone with occasional side chains of another fluorocarbon. The side chain terminates in a sulfonic acid (-SO3H).
With the exception of the sulfonic acid group, Nafion is a fluorocarbon polymer. Like most fluoropolymers, it is extremely resistant to chemical attack (corrosion resistant). The sulfonic acid group is immobilized within the bulk fluorocarbon matrix and cannot be removed, but unlike the fluorocarbon matrix the sulfonic acid groups do participate in chemical reactions. The presence of the sulfonic acid adds three important properties to Nafion:
-
Nafion functions as an acid catalyst due to the strongly acid properties of the sulfonic acid group.
-
Nafion functions as an ion exchange resin when exposed to solutions.
-
Nafion very readily absorbs water, from the vapor phase or from the liquid phase. Each sulfonic acid group will absorb up to 13 molecules of water. The sulfonic acid groups form ionic channels through the bulk hydrophobic polymer, and water is very readily transported through these channels. Nafion functions like a very selective, semi-permeable membrane to water vapor.
The physical properties of Nafion are similar to those of other fluoropolymers. It is translucent plastic. When used as an ion exchange membrane, it is specified by its manufacturer DuPont to operate at temperatures up to 190°C. When used with gases as a dryer, it is specified by to operate at temperature up to 150°C.
The burst pressure of Nafion tubing is generally greater than 200 psig (over 13 bar) but it varies with the diameter and wall thickness. An unusual property of Nafion is its propensity to change in physical size. As Nafion absorbs water, it swells by up to 22%. When exposed to alcohols it increases in size by up to 88%.
How stable is Nafion?
Nafion is made from Tetrafluoroethylene (Teflon), the most chemical-resistant polymer available. Nafion has a strong acid group which reacts with some materials.
These products are nominally trace organic materials present in air which are the result in incomplete combustion or chemical leakage of active products. The appearance of a slight color on the tubing from these organics does not indicate it has lost its drying properties. The dryers should be stored in polyethylene bags to prevent the color change.
Nafion is very stable and will not undergo chemical changes unless exposed to water solutions containing salts. The product is thermally stable up to a temperature of 160°C (329°F). Nafion will not react with any of the usual gases and vapors present in monitoring applications.
Why does Nafion turn brown when stored? Does Nafion have a shelf life?
Over time we have observed that Nafion can turn discolor to a brown hue over time when exposed to atmosphere or bright light. Our tests have shown that this discoloration has no effect on the water transfer properties of the material – so you can install the product and continue to use it without loss of performance.
Similarly, we have found that as long as Nafion is kept in a clean area the performance should not degrade over time. In most applications the Nafion tubing lasts as long as the device, expect when contaminants or dirt present in the sample stream block the water transfer chains on the inside surface of the tubing. In these cases, the product will have a lifetime in the application dependent on the amount of contaminants present.
What are the current uses for Nafion?
Nafion is used primarily as the ion exchange membrane in chlor-alkali production, where salt solutions are separated electrolytically into chlorine and sodium hydroxide. In this environment, Nafion must tolerate elevated temperatures, high electrical currents, and extremely corrosive compounds.
Nafion was developed specifically for this application.
offers a wide variety of gas dryers and humidifiers based on the selective water transport properties of Nafion. Nafion is also used as the active membrane in fuel cells. Its acid properties are exploited to drive acid-catalyzed reactions. Its ion exchange properties are used in numerous applications in scientific instrumentation.
Its conductive properties make it suitable for use as a coating for the tip of pacemaker electrodes, where it resists overgrowth of surrounding tissues while remaining conductive.
I can’t find a product that precisely meets my needs. Do I have any options?
Over 60% of business is done with OEMs (Original equipment manufacturers) who require custom products to meet their design specifications. All tubing production, molding of fittings and assembly is done in house. Typical modifications include custom dryer lengths, proprietary fittings, and custom molded housings.
How is Main Stream involved in Nafion production?
Nafion tubing under exclusive license from DuPont. Secure Supplies and our partners purchases Nafion resin from DuPont, extrudes tubing, and performs a complex chemical treatment to activate it. They exploit the water transport properties of Nafion to produce dryers and humidifiers in a wide range of sizes, from very small research models to large, process versions. Dupont also supplies Nafion tubing for applications other than drying.
How are Nafion dryers different from traditional gas dryers?
Drying is usually accomplished with one of four devices: condensers, desiccant dryers, permeation dryers, or Nafion dryers.
Condensers such as Peltier or Thermoelectric Coolers function by cooling a gas stream until water and other liquids coalesce, then collecting the condensate and draining it away. Condensers are simple to operate.
Unfortunately, they are very non-specific; not only do they remove whatever gases condense at lower temperature, but also at least a portion of whatever gases dissolve in the condensate. Condenser systems are designed to minimize the contact of the gas stream with the condensate to limit this deficiency, but water-soluble gases are always lost to varying degrees depending upon the solubility of the gas in question. Large amounts of gases such as sulfur dioxide are lost by condensers, and condensers are entirely inappropriate for dry gas streams containing hydrogen chloride or chlorine (unless its removal is desired).
Desiccant dryers function by binding water to an absorbent. The absorbent may be a solid (such as silica gel) or a liquid (such as sulfuric acid) that binds water to its chemical structure as water-of-hydration. Desiccants are very simple to operate. Unfortunately, like condensers, they are very non-specific, and remove many compounds other than water.
Unlike with condensers, water cannot be removed from desiccants by simply draining it away. While in operation, desiccants become progressively loaded with water, and must periodically be regenerated by replacement of the desiccant or by driving off the water. Continuous operation desiccant dryers use either a drastic change in surrounding pressure (pressure-swing heatless desiccant dryers) or a drastic change in surrounding temperature (temperature-swing desiccant dryers) to remove water from one chamber of desiccant while a second chamber is used, and the chambers alternate operation and regeneration.
Permeation dryers function on a principle of selection on the basis of molecular size. Permeation dryers are a micro porous material. When forced under pressure across the surface of the micro porous material, large molecules tend to remain in the gas stream while small molecules tend to move through the micro porous material and are removed. Permeation dryers are very simple to operate but are primarily suitable as air dryers. Nitrogen and oxygen are larger molecules than water, so air can be dried by this method. Permeation dryers are too non-specific to dry complex gas sample streams.
Nafion dryers function on a principle of selection on the basis of affinity for the sulfonic acid group. Although water passage through Nafion is described as permeation, Nafion dryers do not operate on the same principles as permeation dryers.
Nafion is not a micro porous material, separating compounds on the basis of their molecular size. For example, Nafion dryers can remove water from a hydrogen stream, even though the hydrogen molecule is much smaller than water. Pressure is not required to drive the process; the driving force for the reaction is the partial pressure of water vapor. Unlike competing methods, Nafion dryers are highly selective in the compounds they remove.
What is the chemical process whereby Nafion tubing dries or humidifies a gas stream?
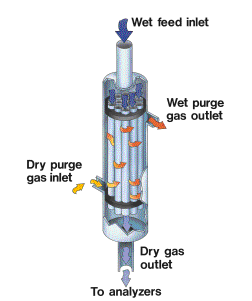
Nafion dryers contain one or more strands of Nafion tubing. Most of the Nafion tubing wall is inert fluorocarbon polymer, and does not participate in the process. Since sulfonic acid is ionic in character and the bulk material is not, the sulfonic acid group within the Nafion tend to clump together.
The activation process for Nafion reorients its sulfonic acid groups together into ionic channels extending from one side of the tubing wall to the opposite side.
When water strikes an exposed sulfonic acid group on the surface of the tubing, the water is initially bound by the surface group. Additional sulfonic acid groups deeper in the wall have less water attached to them, and consequently a higher affinity for water. Water molecules absorbed onto the surface of the tubing are therefore quickly passed on to underlying sulfonic acid groups, until the water reaches the opposite side.
The water molecule then perevaporates into the surrounding medium. This process continues until the water vapor pressure gradient across the tubing wall is eliminated. If a very low water vapor pressure is maintained outside the tubing wall, water will stream across the tubing wall very quickly.
This is a First Order Kinetic reaction, and it proceeds very rapidly. Water is removed from a gas stream directly from the vapor phase, and is released into the surrounding environment directly to the vapor phase. There is no net phase change, and energy is thus not consumed by the process.
What compounds other than water are removed by Nafion? By what mechanism?
When used in contact with solutions (in the liquid phase) Nafion in the form used in dryers functions as an cationic exchange resin, passing not only water but also positively charged ions (cations) from the solution, while resisting the passage of negatively charged ions (anions).
When used in contact with the gas phase, Nafion is much more selective. Ionic compounds do not dissociate into positive and negative ions in the gas phase at the operating temperatures of the dryers, so free negative ions are not available to migrate across the Nafion membrane (tubing wall).
Migration occurs as the result of a different mechanism. Compounds that present an exposed hydroxyl group (-OH) are essentially the only compounds known to migrate through Nafion in the gas phase. This is apparently due to hydrogen bonding with the sulfonic acid groups that are surrounded by the fluorocarbon matrix within Nafion.
Most hydroxides are high-boiling solids (sodium hydroxide, calcium hydroxide, etc.) that are not present as gases within the operating temperature range of the dryers. Only three compounds or classes of compounds are normally removed directly by Nafion dryers:
-
Water (H-OH)
-
Ammonia (when water is present, NH3 reacts to form NH3-OH)
-
Alcohols (R-OH, where R is any organic group)
In addition to these three, certain other organic compounds may also be removed if they can be converted into alcohols. Aldehydes (R-H-C=O) and ketones (R1-R2-C=O) can both undergo a process called enolization (conversion to alcohol or “enol”). The carbonyl group within aldehydes and ketones can be acid catalyzed to react with water to form an alcohol in the following reversible reaction: C=O + H2OHO-C-OH.
Nafion is strongly acidic due to the presence of the sulfonic acid groups.
How is it possible that Nafion tubing can function either as a dryer or as a humidifier?
Nafion functions essentially as a highly selective, semi-permeable membrane to water vapor. If gases inside Nafion tubing are wetter than gases surrounding the tubing, drying will occur. If the surrounding gases are wetter, humidification will occur.
In the simplest case, a strand of Nafion is suspended in ambient air. If the sample stream inside is much wetter than ambient air (such as breath samples), the sample falls to ambient humidity. If the sample stream inside is much drier than ambient air (such as calibration cylinder gases), the sample rises to ambient humidity.
To dry the sample to lower humidity, the surrounding air must be dried. For simple, portable applications, the Nafion tubing is packed in desiccant.
The desiccant provides a very dry surrounding purge environment, while the Nafion tubing provides selectivity in drying. The desiccant gradually saturates with water and periodically must be regenerated or replaced.
For continuous operation, one or more strands of Nafion tubing are suspended within a housing that is purged with a dry gas. For humidification, the housing is filled with water to create a highly humid purge environment.
What are the effects of pressure on Nafion dryers and humidifiers?
Aside from the purely physical effects of pressure on Nafion tubing, total pressure has essentially no effect on Nafion dryers.
Nafion tubing is relatively tough but quite flexible. The tubing has a relatively high burst point when subjected to a positive pressure. Positive pressure inside the tubing causes it to swell slightly, exposing the maximum surface area and slightly improving performance.
Because the tubing is flexible, negative pressure inside the tubing can cause it to collapse like a soda straw.
This collapse will prevent sample flow and cause dryer failure. Negative sample pressure should be limited to 5 inches of water or less if the dryer is heated. Greater negative pressures will constrict the tubing, reducing active surface area and thus reducing performance, or will totally collapse the tubing.
Although total pressure has only physical effects on Nafion performance, the fundamental driving force of the process is the water vapor pressure gradient. Functioning essentially as a semi-permeable membrane to water vapor, Nafion equilibrates the water vapor pressure across the tubing wall. Since doubling the pressure of a sample doubles the partial pressure of the water vapor component of that sample, increased pressure on the sample side of the tubing wall or decreased pressure on the purge side of the sample wall can be used to stimulate the process.
What are the effects of temperature on Nafion dryers and humidifiers?
The effects of temperature on Nafion function are much more complex than the effects of pressure. There are two major effects.
The Primary Effect is a purely kinetic one. Water absorption and transport by Nafion is a First Order Kinetic reaction. As such the rate of reaction is a logarithmic function of temperature.
Within the normal operating temperature range for Nafion dryers/humidifiers, the rate of water absorption roughly doubles for every 10°C rise in operating temperature. Thus, at higher temperatures, the water vapor pressure inside the tubing comes to equilibrium with the outside water vapor pressure faster which means that gases dry or humidify faster.
The Secondary Effect of temperature on Nafion function relates to the final equilibrium point. For drying or humidification to occur, there must be a water vapor pressure gradient across the tubing wall. Drying/humidification stops when there is no longer a gradient; at this point equilibrium has been reached. It might seem that if the water vapor pressure outside the tubing were zero, the water vapor pressure of the sample inside the tubing would eventually fall to zero also. This is unfortunately not the case.
The wall of the tubing always retains some residual water because the sulfonic acid groups within the Nafion polymer never give up all of their water.
This residual water is temperature dependent. At higher temperatures more water is retained within the wall and cannot be removed. This water concentration within the wall corresponds to some water vapor pressure level outside the wall. When the water vapor pressure of the sample falls to a level matching the residual water level within the wall, there is no longer a gradient, equilibrium is reached, and drying stops.
This residual water level within the tubing wall determines the lowest water level (dew point) of the sample achievable by a dryer.
At room temperature (20°C) the residual water in the tubing wall corresponds to a final achievable dew point of about -40°C (about 75 ppm of water). For every one degree C rise in operating temperature above room temperature, the final equilibrium dew point also rises about one degree (C).
The combination of these two effects means that at higher operating temperatures, Nafion dryers initially remove water faster but stop drying (come to equilibrium) at a higher final dew point. For best performance, a temperature gradient should exist down the length of the dryer.
The sample inlet end should be hot to keep water in the vapor phase and to initially remove water very quickly, removing the bulk of the water in a wet sample.
As the sample passes down the length of the dryer, the temperature should be reduced, because the sample contains less water and its dew point is lower so the sample temperature will still remain above its dew point. the sample outlet end should be cool, at room temperature (or lower, if a cooling system is employed) so that the final equilibrium dew point when the sample exits the dryer is as low as possible.
What are the limitations and the most common causes of dryer failure?
Nafion is extremely corrosion resistant. No compounds that exist in the vapor phase within the operational temperature range of Nafion are known to attack it. Even hydrofluoric acid or other concentrated acids are tolerated by Nafion. The corrosion limitations of Nafion dryers and humidifiers are due to the materials used for housings and gas connections. Secure Supplies offers product configurations that will tolerate almost any sample matrix.
Pressure limitations of the dryers and humidifiers are also due to the housings and gas connections. Versions are available that will tolerate up to 150 psig (10 bar), depending upon design.
Although Nafion will tolerate temperatures as high as 190°C, a maximum operating temperature of 150°C for Nafion dryers and humidifiers is recommended. Nafion is a strong acid catalyst and, as operating temperatures rise above 110°C, unwanted chemical reactions may be stimulated within the sample gas matrix. For this reason, most dryer installations operate at 100°C or less.
There is no initial water content limitation imposed by Nafion dryers. The final performance of the dryer depends upon the initial water content, the sample flow rate, and the operating temperature. When sizing a dryer always use the wet gas flow rate, not the flow rate required by the analyzer.
As mentioned previously, ammonia, alcohols, and some other organic compounds that can be converted to alcohols are removed by Nafion dryers. Other gases can be dried without loss of the gas of interest.
To function effectively, both the external and internal surfaces of Nafion tubing must be clear of obstruction. Films of oil or other deposits will degrade dryer or humidifier performance. Over time, deposits will accumulate if the purge air is contaminated with oil, if the sample is inadequately filtered, or if unforeseen chemical reactions occur within the sample that deposit residues within the dryer. Generally, these processes will cause a gradual decline in performance over a period of many months or years and may be reversed by periodic cleaning.
There are two common causes of unexpected Nafion dryer failure, collapse of the Nafion tubing and the introduction of liquid water into the dryer.
-
Collapse of the tubing is caused by negative pressure inside the tubing, commonly caused by pulling the sample stream through the dryer with a pump while pushing the purge gas through the purge housing. To avoid this problem, the pump for the purge gas is placed after the dryer in the sample stream.
-
Introduction of liquid water into the dryer causes failure by an unexpected mechanism. Ordinarily Nafion dryers remove water vapor from the sample and perevaporate it into the surrounding medium.
-
There is no net phase change, and no energy is consumed. If liquid water enters the dryer, it is still absorbed then perevaporated as water vapor. Since energy is thereby consumed, the dryer begins to cool. As it cools, it condenses more water, causing more cooling. There is a cascade failure in which the dryer becomes progressively colder and wetter until it is completely soaked and nonfunctional.
In most instances, when the dryer becomes physically wet, the process can be reversed by simply discontinuing sample flow and permitting the purge gas to dry out the device.
The dryer then recovers its normal performance. Unfortunately, in some instances the sample may contain ionic compounds in the gas phase. If present, these ionic compounds will dissolve in the liquid water accumulating within the dryer. Once present in solution, the ions can participate in ion exchange with the Nafion tubing, converting the tubing to another form that is much less water absorptive.
Should this occur, it will be necessary to regenerate the Nafion tubing by treatment with acid before it fully recovers its normal performance.
If reasonable care is exercised to keep the sample and dryer sufficiently hot to prevent liquid water from entering the dryer, and if excessive negative sample pressure is avoided, the dryer will function indefinitely.
For More Information our our De humidifiers to make De ionised water for Electrolyzers and our In line filtration and gas scrubbing that work live on demand in the gas feed stock lines to any scale items contact
Daniel Donatelli
Secure Supplies

Call
T USA : +1 520 906 2455
T TH : + 66 (0)86 647 3443
© 2009 SECURE SUPPLIES LIMTIED by Daniel Donatelli All Rights Reserved. No duplication is permitted of this site graphically or conceptually unless requested and approved in writing.
All Rights Reserved Worldwide. Copyright © 2012 Secure Supplies Limited Daniel Donatelli © 2009
Copyright Disclaimer Under Section 107 of the Copyright Act 1976, allowance is made for -fair use- for purposes such as criticism, comment, news reporting, teaching, scholarship, and research. Fair use is a use permitted by copyright statute that might otherwise be infringing. Non-profit, educational or personal use tips the balance in favor of fair use.
Please help make the change to clean fuels It is time to order.
Share it First








