Stanley MeyerHHO
#Hydrogen #Electric #Generator

Stanley Meyer's water fuel cell - Wikipedia, the free encyclopedia
Improving IC Engine Efficiency
Today’s efficiency situation:
FUEL 100%
PUSHING THE PISTONS 35% BAD
OVERCOMING ENGINE FRICTION AND PUMPING THE AIR AND FUEL
(typical US driving condition) 20% INsane That is 80% Wastage
Are we stuck with ~20% auto engine efficiency?
What can be done?
Also needed are ways to improve lean flammability in gasoline engines. That is, the ability to burn real lean is limited by the fuel. If the gasoline-air mixture is too lean, the flame will not have enough speed to get across the cylinder in the time permitted by the engine RPM the driver wants, or the flame will not even start – the cylinder misfires, and then the catalyst has to oxidize a huge amount of UHC and thus may overheat (which might mean you have to buy a new catalyst).
Background:
A first course on thermodynamics may teach the efficiency of the Otto cycle (which is the ideal cycle used to simulate the gasoline spark ignition auto engine). Such a course would derive the following equation for the Otto cycle efficiency:
h = 1 – 1/rvg-1
The compression ratio of the engine is rv. Actually, this is a volume ratio. It is the ratio of the volume in a cylinder when the piston is at the bottom of the cylinder to the volume in the cylinder when the piston is at its top position: rv = Vbottom/Vtop.
Most auto engines have compression ratios in the 9 to 10.5 range. We note: the higher the compression ratio, the higher the efficiency! The g parameter is the ratio of the specific heats, ie, the constant pressure specific heat over the constant volume specific heat. In practical terms, the higher the g, the higher the efficiency. A gas such as helium or argon, composed only of atoms, has the highest gpossible, 1.67. Room air on the other hand, being mainly composed of O2 and N2 molecules has a g of 1.4. Fuel vapor has g less than that of air. The mixture of air and gasoline vapor inducted into the engine has a g of about 1.35. As this mixture is compressed and heated during the compression stroke, its g drops to about 1.33. Upon combustion (when the piston is near its top position), the fuel is oxidized to CO2(and some CO) and H2O, and g drops further. It drops into the 1.20-1.25 range. The overall, effective g for the whole cycle for use in the efficiency equation above is about 1.27.
The rule of thumb is: the greater the complexity of the molecules, the lower the g. The lower limit is 1. Argon and helium atoms only translate, that is, they move along straight paths until they encounter another atom. Room air molecules translate and rotate (about 2 of their axes). Hot air starts to vibrate (as two nuclei connected by a spring). Molecules of fuel vapor have a lot of opportunity to vibrate, even at room temperature. The products of combustion vibrate. However, only the translation of the molecules PUSHES the piston. The other modes of molecular motion do nothing for pushing the piston. Thus, as g drops (indicating more vibration of the molecules), h drops. A lean engine (ie, an engine with excess air) has a cooler combustion process and more air relative to fuel than the typical engine with a chemically correct mixture. Thus, its g is higher, and its h is greater.
Plug g = 1.27 into the efficiency equation above, assume rv = 10, and you get h = 0.46. Multiply this by about 0.75 to account for real cycle effects (such as the time it takes to burn, heat losses to the coolant, and exhaust valves that open before the piston fully reaches bottom position) and you have h = 0.35. This is the efficiency (given above) of using the chemical energy of the fuel to push the pistons. Multiply this by the mechanical efficiency of the engine, which accounts for the mechanical friction in the engine and for the air (and fuel) pumping work that has to be done, and you have the final, or overall efficiency of the engine. Of course the mechanical efficiency varies with driving conditions. The higher the RPM of the engine, the greater the friction loss. The more closed the throttle (ie, the farther your foot is off the pedal), the higher the pumping loss. For typical US driving, the resultant overall efficiency of the engine is about 20%. Note, your pedal is not really a gas pedal, it is an air pedal! Add the tranny and real axle mechanical friction losses (or the transaxle friction losses) and the drain of a few essential accessories, and you arrive at a 15% fuel-to-wheel efficiency for the typical auto driven in the US.
However, the diesel engine is not subject to this limitation. It runs at high compression ratio. In part, this explains its high efficiency. It also runs lean, and its pumping work is low, further increasing its efficiency over the gasoline engine. Humankind needs quiet, smoke-free, odor-free diesels!
-
Run the engine fuel-lean, that is, use excess air. It is well known that fuel-lean running improves the efficiency. In the old days, under cruising conditions, the engines always ran lean – about 15% excess air -- this was economical. So what happen to change this? The problem is the three-way (CO, UHC, NOx) catalyst used on engine exhausts. This only works if the engine air/fuel ratio (by mass) is stoichiometric (chemically correct). For gasoline this ratio is 14.6:1. The engine computer, acting in concert with the engine air flow sensor, electronic fuel injectors, and exhaust oxygen sensor, maintains the stoichiometric ratio for most of your driving. Only at this ratio can the catalyst both oxidize the CO and UHC (to CO2 and H2O) and chemically reduce the NOx (to N2). (UHC = unburned hydrocarbons.) What humankind needs is a lean-NOx catalyst. Then we could have increased efficiency and continue to be clean!
-
Higher compression ratio. Here, we are limited by autoignition of the gasoline – knock. That is, if the gasoline engine compression is above about 10.5, unless the octane number of the fuel is high, knocking combustion occurs. This is annoying and if persistent, damage to the engine can occur. Thus, gasoline engines are limited in their efficiency by the inability of the fuel to smoothly burn in high compression ratio engines.
-
We need new cycles put into practical use. An example is the Atkinson cycle. This has a smaller compression ratio than expansion ratio. This means TC is reduced since the burnt gas cool as they expand, making the cycle efficient. We throw away less waste heat via the exhaust.
-
Run the engine at optimum conditions, meaning low friction (modest engine speed) and low pumping work (air throttle more open). Try to approach the "pushing-the-pistons" efficiency of 35%. This already is happening in some stationary piston engines – large, slow, piston engines used at pipeline compressor stations, for example. Also, this is an important characteristic of the engines used in the hybrid gasoline-electric vehicles. Let the gasoline engine in the hybrid gasoline-electric power plant only run with good throttle opening and modest RPM. An example of one type of commercially available hybrid engine (a "parallel" type) is found at:
(http://prius.toyota.com/technology/hybrid.html).
Note the hybrid power plant also recovers some of the kinetic energy of the vehicle, by letting this KE drive an electrical generator (during braking). The electrical energy is stored in the batteries. (Normally, this KE is dissipated as heat in the brakes.) An inverter is used to convert DC electricity from the batteries to AC electricity needed by the electric motor and created by the generator.
______________________________
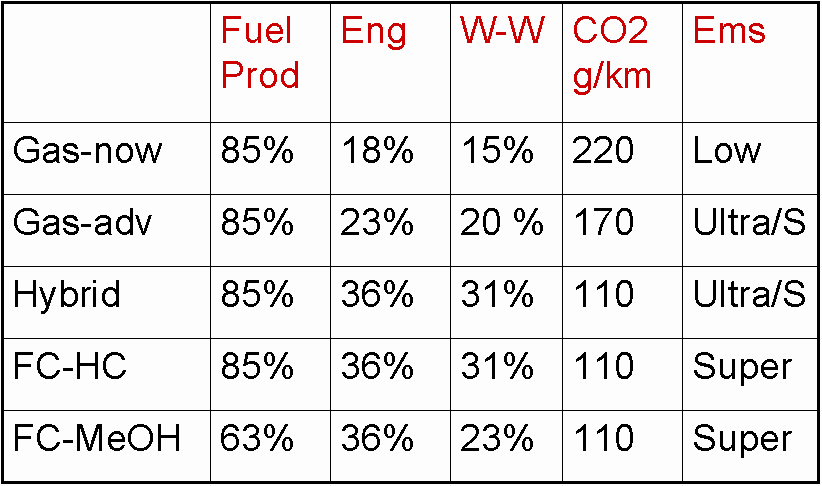
The table below compares the "well-to-wheel" efficiencies of several auto power plants. "Fuel Prod" means the energy efficiency of extracting, refining, and transporting the fuel. "Eng" means the "fuel-to-wheel"efficiency of the vehicle. "Gas" means gasoline engine. "FC-HC" means a PEM fuel cell with a gasoline-to-hydrogen reformer on board. (PEM = proton exchange membrane fuel cell, the fuel cell type that has been getting most of the attention for auto and home use.) "FC-MeOH" means a PEM fuel cell with methanol-to-hydrogen reformer on board.
(The methanol is produced at a refinery by steam reforming natural gas – thus it is a "fossil fuel".) "Ems" means emissions (CO, UHC, NOx) impact. The ratings are "low" (where we are now for autos), "ultra low", and super low".
Call
T USA : +1 520 906 2455
T TH : + 66 (0)86 647 3443
© 2009 SECURE SUPPLIES LIMTIED by Daniel Donatelli All Rights Reserved. No duplication is permitted of this site graphically or conceptually unless requested and approved in writing.
All Rights Reserved Worldwide. Copyright © 2012 Secure Supplies Limited Daniel Donatelli © 2009
Copyright Disclaimer Under Section 107 of the Copyright Act 1976, allowance is made for -fair use- for purposes such as criticism, comment, news reporting, teaching, scholarship, and research. Fair use is a use permitted by copyright statute that might otherwise be infringing. Non-profit, educational or personal use tips the balance in favor of fair use.
Please help make the change to clean fuels It is time to order.
Share it First








